Nitinol Medical Device Simulation Services
We offer expert simulation services involving Finite Element Analysis (FEA) in the engineering and design of Nitinol medical devices and implants. Key areas that simulation can have an impact in device development include:
- Simulate the superelastic behavior of Nitinol devices during complex load cases including bending and twisting.
- Predict the cyclic load response in austenite and martensite phase states of Nitinol components
- Optimize the shape setting process on Nitinol deployed implants including cardiovascular stents
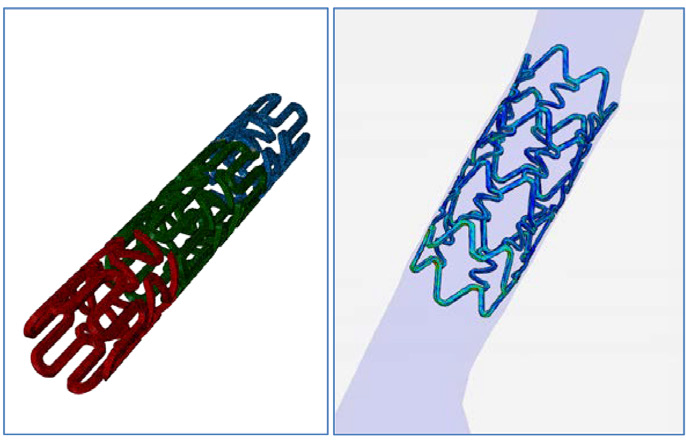
Nitinol stent expansion simulation based on Abaqus
Durability & Fatigue Life Estimation of Nitinol Devices
Class III medical devices, such as a coronary Nitinol stent, are expected to survive throughout the patients lifespan. This can amount to over 600 million cycles under a typical cardiac cycles including twisting and bending that occurs from normal human motions and kinematics. Below are typical durability studies of Nitinol materials where we can conduct simulations:
- Predict the Fatigue Reserve Factor (FRF) from high cycle alternating strains based on the Infinite Life Fatigue Method
- Determine peak, mean and alternating strain tensor quantities for multiple loading conditions including Pulsatile flow and Cardiac Cycles
- Crack initiation and propagation
Nitinol Material Characterization
Nitinol is a technically challenging material due to its ability of phase transformation from austenite to martensite and back to its stress-free austenite state. To model Nitinol correctly, unique material parameters are required from specific test to account for each stage of interaction: Austenite Start (As), Austenite Finish (Af), Martensite Start (Ms) & Martensite Finish (Mf). To help validate the Nitinol properties, we can support a number of tests and standards through FEA:
- Determine and validate the Chronic Outward Force (COF) of Nitinol tubes including stents
- Experimental validation of Nitinol material properties obtained from ASTM F2516
- Determine and validate specific heat & coefficients of thermal expansion from Differential Scanning Calorimetric (DSC) tests
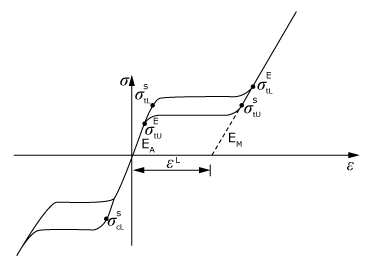
Typical Nitinol Stress-Strain Behavoir
Medical Device Regulatory Support
Nitinol plays an important role in modern medical devices and implants. We work with the Federal Drug Administration (FDA) and understand the regulatory submission requirements. Below are some key areas where we have developed expertise:
- FDA regulatory documentations including:
- Investigational Device Exemptions (IDE)
- Premarket Approval (PMA)
- Premarket Notification [510(k)]
- Model and document worst case conditions and reduce reliance on costly clinical trials
- Simulation-based evaluations of predicate devices for regulatory documentation
Nitinol Use in Medical Device
Nitinol (Ni-Ti-NOL) belongs to a class of Shape Memory Alloys (SMA) made of approximately equal parts Nickel (Ni) and Titanium (Ti). Nitinol, including its name, originated out of the Naval Ordinance Laboratory (NOL) in the early 1960’s. Its a superelastic material that is history dependent and can fully recover its stress induced martensite transformation back to its neutral austenite state. This fully reversible phenomena of Nitinol creates opportunities for designers to develop less invasive surgical devices allowing for faster patient recovery. Medical grade Nitinol is also ideal for devices that undergo repetitive deformations with strains as high as 10% during deployment. Examples of devices that currently benefit from Nitinol unique characteristics include vascular stents, guidewires, blood clot filters, orthopedic implants and a growing number of other class II & III devices.
Contact Us
To contact us regarding a consulting project on a Nitinol medical device with one of our engineers, please fill out the form and we will reach out to you soon.
